Why Novoheart
See What Makes Us Stand Out
Novoheart is a global stem cell biotechnology company dedicated to engineering bioartificial human heart prototypes using state-of-the-art stem cell and bioengineering approaches.

So... Why Are We Here?
In 2022, legislation was passed with the signing of the FDA Modernization Act 2.0 to promote the use of human-based technologies as superior replacements for animals in drug discovery and development. This was exciting news for Novoheart, since our team already produces human-based drug screening products ready for the market.
Revolutionizing Drug Discovery
Capitalizing on our pioneering expertise in pluripotent stem cell biology and bioengineering, we have developed and advanced other mini-Organ systems such as liver, immune, vascular, lung, gut, and bile, as part of the mini-Life Platform. These are linked and driven by our pumping mini-Heart Technology for the most comprehensive human-based screening systems, reducing cost and time, and improving efficacy and predictivity.
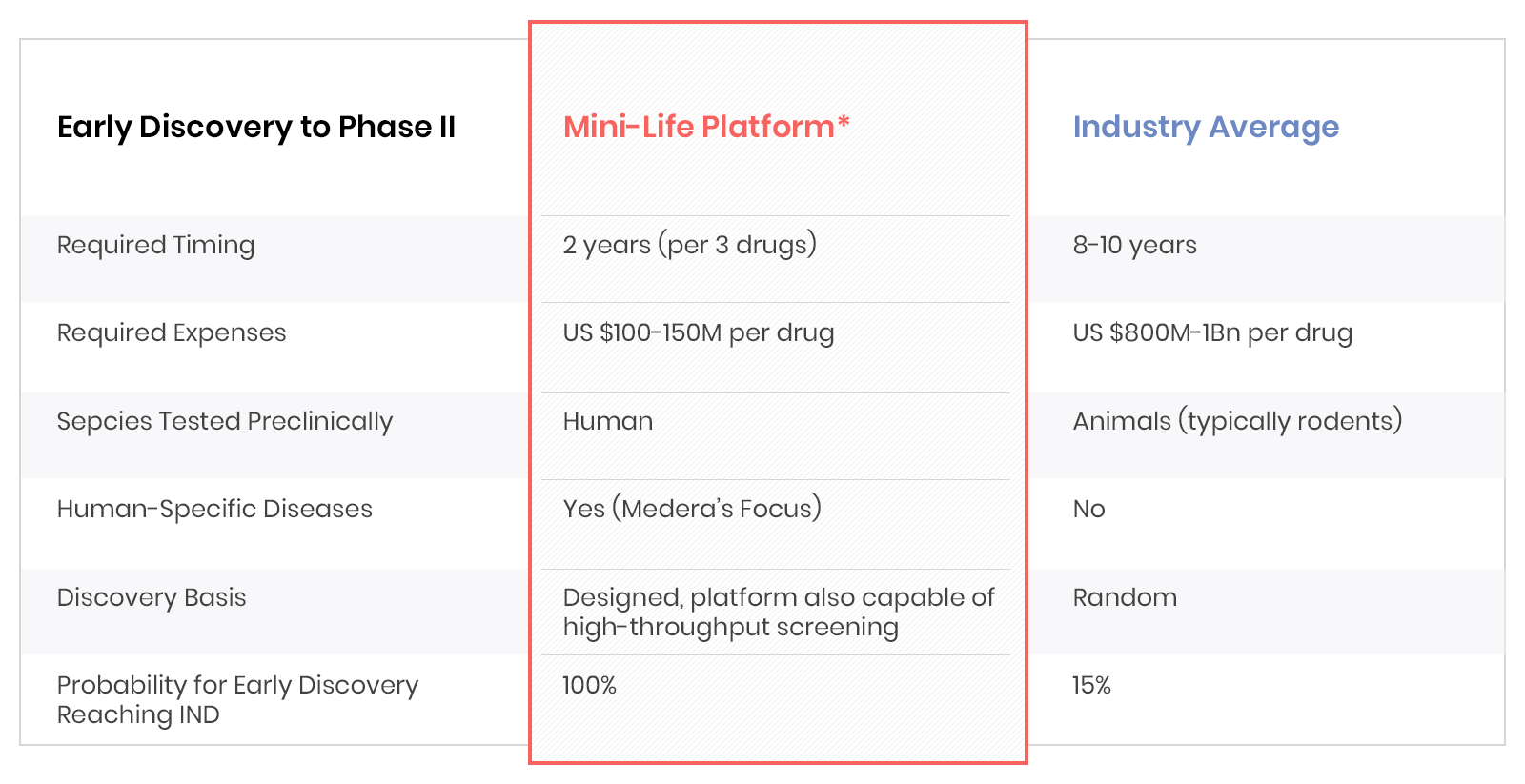
Novoheart is Revolutionizing Drug Discovery
The discovery and development of novel drugs and therapeutics for heart diseases continues to be an unacceptably inefficient process. A major reason is the lack of appropriate human models to simulate the normal and pathological human heart. Traditional animal models are accessible but major species differences in the cardiac anatomy and function exist. What works in animals may be ineffective or even lethal in humans. Likewise, potentially blockbuster drugs for patients never even make it to clinical trials if they are found to be toxic in animals.
Our Scientists are Reducing Patient Harm
Development of drugs and therapeutics is both risky and costly. Numerous drugs have been withdrawn from the market because of adverse clinical side effects and risks to patients. Cardiotoxicity is a common leading cause, even for drugs not intended for the heart. For example, the Cardiac Arrhythmia Suppression Trial (CAST) found several “anti-arrhythmics” to unexpectedly increase mortality by causing lethal arrhythmias. With Novoheart’s proprietary technology, the pro-arrhythmic properties of failed CAST drugs could be readily detected (Shum et al. 2016, Advanced Materials).
Relentless Innovation is in our DNA
In addition to patient health, a single late-stage drug failure typically costs 10+ years of development and an economical investment of USD 2-3 billion. Not only does early detection minimize potential patient harm, it also enables resources to be focused on post-discovery fine-tuning for maximizing benefits and successes.

